When 1 + 1 ≠ 2: How mixed water contaminants rewrite toxicity rules
GA, UNITED STATES, December 23, 2025 /EINPresswire.com/ -- Chemical contaminants in drinking water rarely occur alone, yet risk assessments typically assume their toxic effects simply add up. New research shows this assumption can be misleading. By examining mixtures of common pesticides and disinfection by-products, the study reveals that interactions between chemicals can amplify or suppress toxicity depending on concentration and combination. In some cases, these interactions override the intrinsic toxicity of individual compounds, dramatically altering which chemicals drive overall harm. The findings demonstrate that mixture toxicity is shaped not only by what contaminants are present, but also by how they interact biologically. This work highlights the need for new strategies to identify which chemicals truly matter most for protecting long-term drinking water safety.
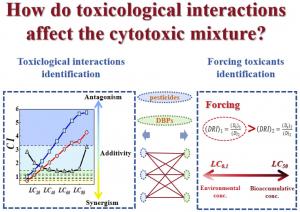
Pesticides and disinfection by-products are widely detected together in treated drinking water at trace levels worldwide. Both groups are associated with long-term health risks, including cytotoxicity and genotoxicity, raising concern about chronic exposure. Current regulatory prioritization largely relies on additive toxicity assumptions, ranking chemicals by their individual toxicity-weighted concentrations. However, growing evidence suggests that chemical mixtures frequently exhibit nonadditive interactions—such as synergy or antagonism—that can distort toxicity predictions. These interactions may cause standard assessment methods to either underestimate or overestimate actual biological effects. Based on these challenges, there is a clear need to investigate mixture-specific toxicological interactions and develop improved frameworks for prioritizing contaminants in drinking water.
In a study published (DOI: 10.1007/s11783-025-2084-6) online on September 15, 2025, in Frontiers of Environmental Science & Engineering, researchers from Sun Yat-sen University and collaborating institutions systematically examined how mixtures of pesticides and disinfection by-products affect mammalian cell toxicity. Using a combination-index framework and chronic cytotoxicity assays, the team analyzed nine binary mixtures across environmentally relevant and higher exposure concentrations. Their work identifies which chemicals act as “forcing toxicants” under different conditions, offering a more realistic approach to ranking contaminants that co-occur in drinking water systems.
The researchers focused on three widely detected pesticides—malathion, chlorothalonil, and deltamethrin—and three nitrogenous disinfection by-products: chloroacetonitrile, bromoacetonitrile, and iodoacetonitrile. Using Chinese hamster ovary cells as a biological endpoint, they measured toxicity across single chemicals and nine pesticide–by-product mixtures.
Results showed that mixture toxicity was strongly shaped by interaction type. Some combinations shifted from additive or mildly synergistic effects at low concentrations to strong antagonism at higher doses. In chlorothalonil–by-product mixtures, antagonistic interactions suppressed overall toxicity by up to two orders of magnitude compared with additive predictions, effectively masking differences among individual by-products.
By calculating dose reduction indices, the team identified which compound dominated toxicity under different exposure scenarios. At environmentally relevant low concentrations, pesticides were the main drivers in most mixtures. At higher concentrations, however, dominance shifted in certain combinations, with either pesticides or disinfection by-products becoming the forcing toxicant. These concentration-dependent shifts demonstrate that toxicity cannot be inferred from single-chemical data alone. Instead, interaction dynamics determine whether a mixture becomes more or less harmful than expected, challenging conventional contaminant ranking approaches.
“Assuming additivity can seriously misrepresent the real toxicity of chemical mixtures,” said the study’s corresponding author. “Our results show that interaction effects can overwhelm intrinsic toxicity, sometimes reducing or exaggerating risk by orders of magnitude. This means that chemicals traditionally viewed as high priority may not always drive toxicity when mixed with others, while less emphasized compounds may become critical under certain conditions. Incorporating interaction analysis allows us to better identify which contaminants truly require control in complex water systems.”
These findings have important implications for drinking water regulation and risk management. Rather than relying solely on single-chemical toxicity rankings, regulators may need mixture-aware frameworks that account for interaction type and exposure concentration. Such approaches could help allocate limited monitoring and treatment resources more effectively, targeting contaminants that actually drive biological risk under realistic conditions. Beyond drinking water, the framework may also apply to other environmental systems where chemical mixtures are unavoidable. Ultimately, recognizing that “more toxic” does not always mean “more dangerous” could lead to more accurate health protection strategies in an increasingly complex chemical world.
DOI
10.1007/s11783-025-2084-6
Original Source URL
https://doi.org/10.1007/s11783-025-2084-6
Funding Information
This project was supported by the National Natural Science Foundation of China (Nos. 52370020 and 52000184), and the Zhuhai Basic and Applied Basic Research Foundation, China (No. 2220004002894).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.